"EBP" refers to "Evidence-based Practice". It is defined as "the conscientious, explicit, and judicious use of current evidence in making decisions about the care of individual patients".
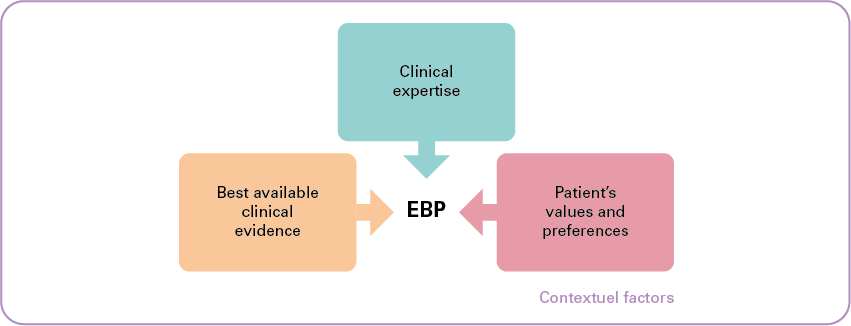
As illustrated in the diagram above, the primary objective of EBP is to combine:
- individual clinical expertise;
- with the best available clinical evidence from systematic research;
- while taking into account the patient's values and preferences.
A fourth dimension is added, namely "contextual factors". These are the elements (such as cost and resource availability) that potentially affect or hinder the strength of a recommendation or the implementation of a guideline.
EBP plays a major role in health policy in two main areas:
- it contributes to improving the quality of care in terms of effectiveness and efficiency, and
- it helps to keep health care spending under control.
Healthcare practice is increasingly based on scientific evidence, which is constantly evolving. Staying informed of the latest science is a real challenge for providers. Clinical practice guidelines and other EBP materials are developed and disseminated to support healthcare practitioners in this process.
This involves the participation of various stakeholders, who collect and disseminate reliable information, evaluate these materials, share knowledge through training, etc.
It is to ensure the coordination of these initiatives that the EBP Network was created in 2018.
The Network combines the expertise of Core Partners[1] with that of stakeholders such as professional and patient associations; they are all represented on an advisory board.
The strategic unit of the Minister of Public Health, the FPS Public Health, NIHDI and the FAMHP are actively involved in the EBP Network via the steering committee. They participate by providing funding and contributing to the definition of strategic directions.
The activities of the Belgian EBP network respect the EBP life cycle.
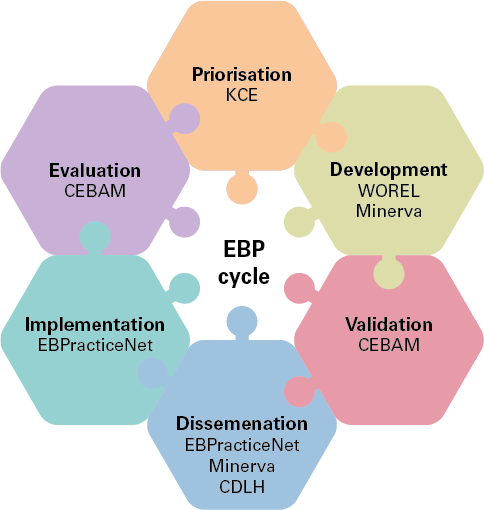
- Prioritisation: the objective is to select the priority topics and propose a strategic approach to the different EBP activities.
- Development: the objective is to maintain or increase the quality and accuracy of EBP products and develop new EBP products for Belgian users.
- Validation: the objective is to evaluate the scientific and methodological validity of the EBP products developed. The approval of the Validation Unit guarantees the quality, precision, appropriateness and validity of EBP products for the Belgian context; it is a prerequisite for the dissemination of clinical practice guidelines within the EBP network.
- Dissemination: the objective is to actively disseminate clinical practice guidelines and other validated EBP products to various users.
- Implementation: the objective is to stimulate the implementation of EBP principles and increase the adoption of EBP products.
- Evaluation: the objective is the development, selection, implementation, and monitoring of procedures for evaluating the implementation, compliance, and/or impact of clinical practice guidelines or other EBP products disseminated through the EBP Network.
For further information on EBP: www.health.belgium.be
[1]Such as KCE, WOREL (Working Group for the Development of Primary Care Recommendations), Minerva, CDLH (Cebam Digital Library for Health), Ebpracticenet and CEBAM (Belgian Centre for Evidence-Based Medicine)