- Details
As seen previously, the consultative bodies play an essential role. Based on their recommendations, the training of healthcare professionals is modified and adapted to scientific and technological developments, as well as to the needs of the population.
Based on the recommendation of the councils, it is possible, for example, to extend the competences of certain professions when they deem it necessary. This sometimes goes hand-in-hand with an extension in the duration of studies.
A quality control can also take place through an obligation of continuous training throughout the professional practice, so that their knowledge is regularly updated. Medicine and the healthcare field are constantly evolving, so it is important that the care provided changes accordingly.
The consultative bodies can therefore give their recommendation on a multitude of subjects concerning the quality of care or the quality of training. The people who sit on these bodies include a large number of professionals who carry out their daily activities in addition to this mandate. They are therefore in a better position than anyone else to see what changes need to be made to maintain the best possible level of quality.
- Details
Another guarantor of the quality of the care provided is the Health Care Quality of Practice Act of 22 April 2019 (hereafter Quality Act).
Its implementation is still in progress. This involves the carrying out of various projects to make this act a reality. These projects include:
| Federal Control Commission |
|
|
| Register of practices |
|
|
|
Portfolio
|
|
|
| Computerised patient record |
|
|
| Cooperation agreements for general practitioner out of hours services |
|
<[1]Law of 22 August 2002 concerning the Rights of Patients
- Details
The inspectors
The health inspector has always played a key role in monitoring the abilities of health professionals and the quality of practice. This function has existed since the Health Act was enacted on the 1st September 1945.
Since 1 June 2019, two inspectors, one French-speaking and one Dutch-speaking, have been dedicated full-time to the supervision of health professionals and the protection of public health. They are called "Healthcare professional medical inspectors".
There are also other types of health inspectors. Some are competent to respond in the event of contagious diseases and infections, while others are dedicated full-time to emergency medical assistance activities.
The Federal Control Commission
The Medical Commissions are an ancient institution, predating the foundation of the Belgian State. With the evolution of legislation, notably the entry into force of Royal Decree No. 78 (1967), they gradually lost their competence in the accreditation of professionals and the recognition of practitioners from abroad.
However, the Commissions have remained competent for monitoring the physical and mental abilities of health professionals and the protection of public health. Their missions included the following:
- temporarily revoke or limit the endorsement of a health care professional when it is determined that the individual's continued practice raises concerns about serious consequences for patients or public health
- contribute to public health and prevent or combat diseases subject to quarantine and communicable diseases
- ensure that the healthcare professions are practised in accordance with the laws and regulations
- investigate and report to the prosecutor's office cases of illegal practice of healthcare professions
- withdraw or limit the endorsement of a healthcare professional who no longer has the physical or mental capacity to continue to practise safely
- etc.
The Quality Act has modernised the organisation of these entities. The Medical Commissions have been replaced by a single, centralised Federal Control Commission, organised into language chambers. Like the Medical Commissions, it is responsible for controlling the physical and mental abilities of professionals.
In addition to the tasks taken over from the former Medical Commissions, new responsibilities have been added.
They include:
- the Quality Act entrusts the Commission with monitoring compliance with quality criteria for the practice of healthcare professionals
- the Control Commission has the power to temporarily ban professionals who represent a danger to patients or public health from practicing
The Federal Supervisory Commission consists of one or more French-speaking and one or more Dutch-speaking chambers. These language chambers take the necessary decisions within the framework of the requirements of the Quality Act on the basis of records or data.
The medical inspectors investigate these files if necessary. In this respect, their investigative powers have been strengthened. Inspections can also be carried out by inspectors of the Federal Agency for Medicines and the control and evaluation service of NIHDI. The Minister of Health also has the power of injunction to investigate facts.
All these measures have been put in place to protect public health. When a health care professional is no longer able to practise, for physical or mental reasons, it is imperative to be able to control the extent to which their activity must be limited or prohibited in order to guarantee patient safety. Furthermore, when a health professional's practice no longer meets quality standards, they must start an improvement process.
Some of the measures of the "Quality Act" are already in place, and others are still being implemented. The long-term goal is to adopt a more modern, transparent and effective monitoring policy than previously, in consultation and collaboration with all healthcare stakeholders.
CASES HANDLED BY THE FEDERAL COMMISSION IN 2020
|
DUTCH CHAMBER |
FRENCH CHAMBER |
The graphs above illustrate the number of cases processed by the two chambers of the Federal Commission in 2020 and the distribution of these cases by region. These numbers vary from year to year depending on a variety of factors. In their reports, the chambers may include other relevant details, such as:
- the number of files by profession
- the number of penalties
- the type of records (physical or psychological aptitude assessment, illegal practice, etc.)
- the records based on existing risk to the patient or public health
- etc.
- Details
Specialist physicians
On completion of basic medical training, the new professional may undergo additional theoretical and practical internship to become certified in a particular field. The theoretical part takes place in practice within the universities.
The practical internship takes place with internship supervisors approved by the FPS Public Health (see internship supervisor approval section above). Depending on the specialties, this additional training can last between three and six years, at the end of which the specialist physician in training can request approval in the desired specialty (e.g. orthopaedic surgery, etc.). After this specialisation, the specialist physician can take further training to acquire an additional qualification such as intensive care or clinical haematology.
Specialist physicians in training are called MSFs.
FUNDING FOR PRACTICAL INTERNSHIP
Since the conclusion of a collective agreement in May 2021, all MSFs have the same minimum basic pay in hospitals in Belgium. Their salaries are funded as follows:
- by the fees they generate by performing procedures;
- by the funding allocated to hospitals in the budget of financial resources.
In addition, the internship supervisor also receives compensation for the educational component of the internship, via NIHDI.
For more information about remuneration: www.health.belgium.be
QUALITY OF THE PRACTICAL INTERNSHIP
The MSF internship quality project is being conducted in several phases. Its ultimate goal is to improve the quality of internship, in particular through the implementation of a new internship quality monitoring system.
ANALYSIS OF THE QUALITY SYSTEM IN DIFFERENT COUNTRIES
The Federal Centre of Health Care Expertise (KCE) published a report in 2010 entitled "Critères de qualité pour les lieux de stage des candidats-médecins généralistes et candidats-spécialistes". This report compares the quality system of internship sites in Belgium with those in France, Canada, the United Kingdom, Switzerland and the Netherlands.
To incorporate the innovations made since 2010, the training quality assessment and improvement project team conducted a similar exercise comparing the quality systems of these different countries.
It was concluded that Belgium has taken few concrete steps towards a quality system, while other countries are still developing. The best practices from other countries will be used in the development of a new quality system.
E-BROCHURE FOR MSFS
In March 2022, the FPS launched an e-brochure for MSFs. It aims to inform MSFs of the complex division of competencies, the legislation and the structures available in the event of problems during internship.
It provides support and answers questions that MSFs often face during their internship.
Consult this brochure: www.macs-aso.be
SURVEYS
Key players in training were interviewed to help conceptualise a quality system. They included MSFs, internship supervisors and coordinating internship supervisors.
These questionnaires allowed us to evaluate the current situation and have a vision of the future of the internship.

The questions were based on the existing legislation and the criteria of the WFME (World Federation of Medical Education), as well as on the systems of other countries and feedback from the representatives of the assistants' associations and the members of the "Medical Specialists" working group of the Superior Council of Specialist Physicians and General Practitioners.
The results, collected in June 2022, were analysed throughout the summer. A report is being prepared and will be sent to the Minister of Health's office at the end of the year with recommendations. This report will then be published.
THE FUTURE OF MSF INTERNSHIP
Based on the survey results, a step-by-step plan will be developed to improve the quality of training. This will also require the adaptation of the current legislation.
- Details
"EBP" refers to "Evidence-based Practice". It is defined as "the conscientious, explicit, and judicious use of current evidence in making decisions about the care of individual patients".
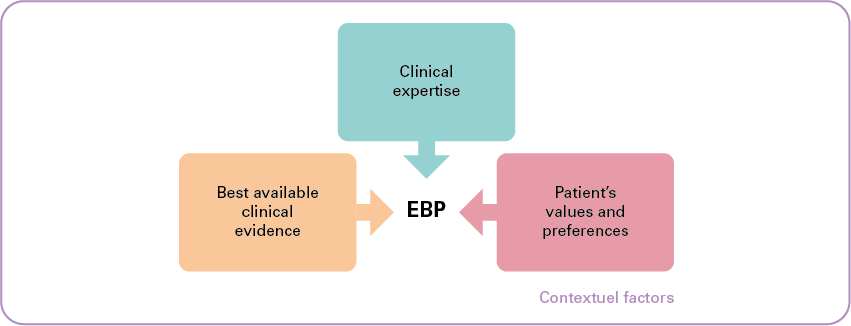
As illustrated in the diagram above, the primary objective of EBP is to combine:
- individual clinical expertise;
- with the best available clinical evidence from systematic research;
- while taking into account the patient's values and preferences.
A fourth dimension is added, namely "contextual factors". These are the elements (such as cost and resource availability) that potentially affect or hinder the strength of a recommendation or the implementation of a guideline.
EBP plays a major role in health policy in two main areas:
- it contributes to improving the quality of care in terms of effectiveness and efficiency, and
- it helps to keep health care spending under control.
Healthcare practice is increasingly based on scientific evidence, which is constantly evolving. Staying informed of the latest science is a real challenge for providers. Clinical practice guidelines and other EBP materials are developed and disseminated to support healthcare practitioners in this process.
This involves the participation of various stakeholders, who collect and disseminate reliable information, evaluate these materials, share knowledge through training, etc.
It is to ensure the coordination of these initiatives that the EBP Network was created in 2018.
The Network combines the expertise of Core Partners[1] with that of stakeholders such as professional and patient associations; they are all represented on an advisory board.
The strategic unit of the Minister of Public Health, the FPS Public Health, NIHDI and the FAMHP are actively involved in the EBP Network via the steering committee. They participate by providing funding and contributing to the definition of strategic directions.
The activities of the Belgian EBP network respect the EBP life cycle.
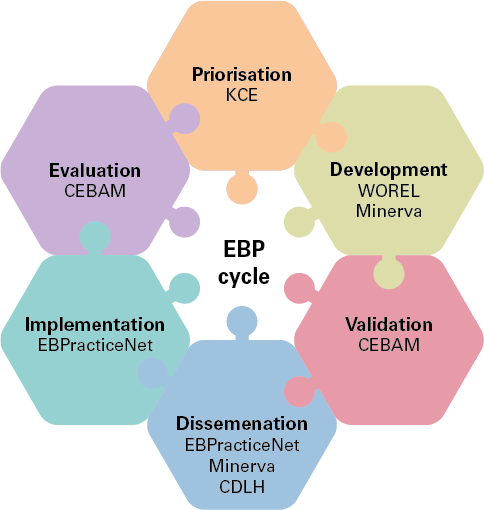
- Prioritisation: the objective is to select the priority topics and propose a strategic approach to the different EBP activities.
- Development: the objective is to maintain or increase the quality and accuracy of EBP products and develop new EBP products for Belgian users.
- Validation: the objective is to evaluate the scientific and methodological validity of the EBP products developed. The approval of the Validation Unit guarantees the quality, precision, appropriateness and validity of EBP products for the Belgian context; it is a prerequisite for the dissemination of clinical practice guidelines within the EBP network.
- Dissemination: the objective is to actively disseminate clinical practice guidelines and other validated EBP products to various users.
- Implementation: the objective is to stimulate the implementation of EBP principles and increase the adoption of EBP products.
- Evaluation: the objective is the development, selection, implementation, and monitoring of procedures for evaluating the implementation, compliance, and/or impact of clinical practice guidelines or other EBP products disseminated through the EBP Network.
For further information on EBP: www.health.belgium.be
[1]Such as KCE, WOREL (Working Group for the Development of Primary Care Recommendations), Minerva, CDLH (Cebam Digital Library for Health), Ebpracticenet and CEBAM (Belgian Centre for Evidence-Based Medicine)